Antimalarials
Buy antimalarial tablets/capsules from our online doctor and pharmacy service delivered direct to you.
Read medical information and answer medical questions to buy treatment online.
Selecting treatment
PricesAll medication supplied is UK licensed.
Prices
| Malaria pill type | Quantity (trip length) | Cost |
|---|---|---|
| Malarone Adult (Generic) | 16 tablets (1 week trip) | £23.20 |
| Malarone Adult (Generic) | 23 tablets (2 week trip) | £33.50 |
| Malarone Adult (Generic) | 30 tablets (3 week trip) | £43.50 |
| Malarone Adult (Generic) | 37 tablets (4 week trip) | £53.65 |
| Malarone Adult (Generic) | 44 tablets (5 week trip) | £66.00 |
| Malarone Adult (Generic) | 51 tablets (6 week trip) | £73.95 |
| Malarone Adult (Generic) | 65 tablets (8 week trip) | £97.50 |
| Malarone Adult (Generic) | 93 tablets (12 week trip) | £134.85 |
| Malarone Adult (Generic) | 193 tablets (6 month trip) | £279.85 |
| Malarone Adult (Glaxo branded) | 16 tablets (1 week trip) | £46.90 |
| Malarone Adult (Glaxo branded) | 23 tablets (2 week trip) | £63.10 |
| Malarone Adult (Glaxo branded) | 30 tablets (3 week trip) | £79.30 |
| Malarone Adult (Glaxo branded) | 37 tablets (4 week trip) | £95.50 |
| Malarone Adult (Glaxo branded) | 44 tablets (5 week trip) | £114.40 |
| Malarone Adult (Glaxo branded) | 51 tablets (6 week trip) | £132.60 |
| Malarone Adult (Glaxo branded) | 65 tablets (8 week trip) | £170.10 |
| Malarone Adult (Glaxo branded) | 93 tablets (12 week trip) | £218.55 |
| Malarone Adult (Glaxo branded) | 193 tablets (6 month trip) | £450.00 |
| Doxycycline 100mg | 37 capsules (1 week trip) | £11.80 |
| Doxycycline 100mg | 44 capsules (2 week trip) | £12.80 |
| Doxycycline 100mg | 51 capsules (3 week trip) | £13.80 |
| Doxycycline 100mg | 58 capsules (4 week trip) | £14.90 |
| Doxycycline 100mg | 65 capsules (5 week trip) | £15.90 |
| Doxycycline 100mg | 72 capsules (6 week trip) | £16.90 |
| Doxycycline 100mg | 86 capsules (8 week trip) | £19.20 |
| Doxycycline 100mg | 114 capsules (12 week trip) | £23.60 |
| Doxycycline 100mg | 212 capsules (6 month trip) | £42.00 |
| Doxycycline 100mg | 400 capsules (1 year trip) | £75.00 |
| Mefloquine 250mg (Lariam) | 8 tablets (1 week trip) | £22.90 |
| Mefloquine 250mg (Lariam) | 9 tablets (2 week trip) | £24.10 |
| Mefloquine 250mg (Lariam) | 10 tablets (3 week trip) | £25.90 |
| Mefloquine 250mg (Lariam) | 11 tablets (4 week trip) | £28.10 |
| Mefloquine 250mg (Lariam) | 12 tablets (5 week trip) | £30.10 |
| Mefloquine 250mg (Lariam) | 13 tablets (6 week trip) | £32.10 |
| Mefloquine 250mg (Lariam) | 15 tablets (8 week trip) | £35.90 |
| Mefloquine 250mg (Lariam) | 19 tablets (12 week trip) | £48.10 |
| Mefloquine 250mg (Lariam) | 32 tablets (24 week trip) | £79.00 |
| Mefloquine 250mg (Lariam) | 58 tablets (52 week trip) | £135.00 |
Price match guarantee
Prescription issued online - small prescription fee per order.
Prescription fees
Dr Fox supplies medicine on prescription and charges a small prescription fee based on the order value of each prescription.
Prescriptions are issued by our doctors online and sent electronically to our pharmacy.
| Order value | Prescription fee |
|---|---|
| up to £10 | £1.00 |
| up to £20 | £2.00 |
| up to £40 | £3.00 |
| over £40 | £4.00 |
If you have your own private or NHS paper prescription please post to our pharmacy (details).
Dr Fox prices are 25%–50% lower than other UK online clinics.
Delivery charges
UK delivery only: £2.90 per consultation via Royal Mail Tracked 24 Signed For (1-3 working days with tracking).
Parcel forwarding services are not permitted. Use only UK home or work delivery address.
Returns and refunds - unwanted items can be returned within 14 working days for a full refund.
Medical information
Written and reviewed by a team of doctors. Dr Fox is regulated by the CQC & GPhC.
Background
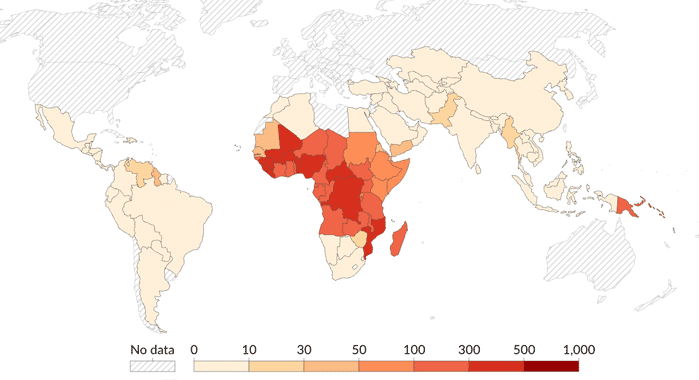
Every year more than 1,500 travellers from the UK catch malaria and several of them die. The onset of serious illness can be rapid. The right antimalarial provides good protection. The risks are higher for children and pregnant women, and for people with ongoing medical conditions.
Do NOT forget you may also need vaccines and other health advice from a GP or travel clinic.
Which antimalarial to buy online?
No one type of recommended antimalarial works better than another. Some are daily tablets or capsules, some weekly. Some are started a few days before travel, others 10 days before. Some are continued for a month after returning home, others for only a week.
Dr Fox offers Malarone (atovaquone/proguanil), Lariam (mefloquine), and doxycycline. These are now the most commonly recommended malaria prevention medications.
Compare different antimalarials
How to take antimalarials (tablets or capsules)
- Take the right antimalarial for the area you are going to.
- Start your tablets/capsules before entering a malaria zone. This may be a few days or up to 10 days before.
- Take the tablets/capsules absolutely regularly, preferably with or after a meal.
- Continue to take them for 4 weeks after leaving the malaria area. This period is reduced to 7 days for Malarone (atovaquone/proguanil).
- No antimalarial is 100% effective. Malaria risk can also be reduced by avoiding mosquito bites (details below).
Where are you travelling?
Selecting your destination above opens a country information page on the NHS Fit For Travel website. This page contains health information including vaccines and malaria precautions for that country. The 'malaria map' link shows high risk and low risk areas within that country (sometimes at different times of the year), and the 'Malaria' section includes more information including which malaria prevention tablets/capsules (prophylaxis) are recommended.
Antimalarial recommendations are based on malaria parasite (Plasmodium) drug resistance. It is important to take the medicine which is recommended for the area being visited. Drug resistance is becoming an increasing problem with the malaria parasite. The older preventative regimes including chloroquine, proguanil, and a combination of the two are now rarely effective, and are not generally prescribed.
Avoiding mosquito bites
Malaria is transmitted by mosquitoes which are themselves infected with the plasmodium parasite. Avoiding mosquito bites is an essential part of protecting yourself from malaria and some other tropical diseases, e.g. dengue fever, yellow fever, zika.
Mosquitoes can bite at any time of day. Most bites by malaria mosquitos occur in the evening and overnight between dusk and dawn. In contrast, dengue fever mosquitoes tend to bite during the day.
- Wear long-sleeved clothing and long trousers if you are out at dusk, dawn, and at night. Several companies sell insect resistant travel clothing pre-treated with insecticide (permethrin).
- Use insect repellent containing DEET on exposed skin and under thin clothing, particularly around the ankles. The best strength DEET is 50% - there is no added benefit to using higher concentrations. Other repellents containing picaridin, IR3535, or lemon eucalyptus are less effective than DEET and must be reapplied very frequently.
- Insect repellent room sprays, mosquito coils, and heating insecticide impregnated tablets all reduce the risk of bites, and should be used to kill mosquitoes in bedrooms before going to bed.
- Where possible sleep in screened rooms and use a mosquito net, preferably one impregnated with insecticide (permethrin). Mosquitos are deterred by air conditioning but not fans.
- Ultrasound devices, mobile phone apps, garlic, vitamin B, marmite, homeopathic products, tonic water, alcohol, tea tree oil, and citronella DO NOT prevent bites.
For further information see Travel Health Pro - Insect and tick bite avoidance and NHS - Malaria Prevention.
What are the symptoms of malaria?
Malaria symptoms start out similar to flu. Symptoms include fever, shivers, sweating, backache, joint pains, headache, vomiting, diarrhoea, cough, and sometimes delirium.
These symptoms may take a week or more to develop after you have been bitten by an infected mosquito.
- Seek medical advice if you get malaria symptoms for up to a year after exposure, even after taking antimalarials. Malaria is diagnosed with a simple blood test.
- If you are travelling in remote areas for prolonged periods it may be helpful to also carry a malaria treatment and/or a malaria testing kit with you. Discuss this with your regular doctor or a specialist travel clinic.
Pregnancy and malaria
Pregnant women are at a much higher risk of developing malaria and becoming very seriously ill with a higher risk of death for themselves or their unborn child. Pregnant women are strongly advised not to travel to a malaria zone, but if travel is completely unavoidable, they should take preventative medication and do all they can to avoid mosquito bites. The risk from malaria is much greater than any risk to the baby from taking antimalarial medication.
Taking Lariam
Most people can take Lariam (mefloquine) with no problems. However in a few people it can cause significant and serious side effects, which may last for several months after stopping. Suitability for taking Lariam is assessed in the online consultation. There is a patient alert card within the medicine pack which should be carried whilst taking Lariam. For further details see the Lariam Patient Leaflet.
There is a very rare theoretical risk of abnormal heart rhythm if Lariam (mefloquine) is taken at the same time as some other medications - please check this list.
Buy treatmentAntimalarial tablets/capsules are supplied on prescription - our doctors will issue the prescription online direct to our pharmacy. Dr Fox Pharmacy supplies medicines only for adults (over 18 years). Further information about malaria tablets for children.
Comparing antimalarials
| Doxycycline | Malarone (generic & branded) | Lariam (mefloquine) | |||
|---|---|---|---|---|---|
| Brand name | |||||
| Many different | Malarone (GSK) or non-branded | Lariam | |||
| Dosage | |||||
| 100mg capsule. One capsule daily with food | Combined tablet 250mg atovaquone/100mg proguanil. One tablet daily with food | 250mg tablet. One tablet weekly on the same day each week | |||
| When to start before entering malaria zone | |||||
| 2 days before | 2 days before | 10 days before | |||
| When to stop after leaving malaria zone | |||||
| 4 weeks after | 7 days after | 4 weeks after | |||
| Number of tablets/capsules for 1 week trip | |||||
| 37 capsules | 16 tablets | 8 tablets | |||
| Side effects and cautions | |||||
| Intestinal upset, vaginal thrush, heart burn. Increased skin sensitivity to bright sun. | Intestinal upsets, headache. | Intestinal upset, headache, loss of balance, dizziness. Less common: sleep and mental disturbance. Caution driving or operating machinery. | |||
| Duration of long term use | |||||
| 2 years | 1 year | 3 years | |||
| Manufacturer's Patient Information Leaflet (PDF) | |||||
| Leaflet | Leaflet | Leaflet | |||

Authored 09 January 2010 by Dr Tony Steele
MB ChB Sheffield University 1983. Former hospital doctor and GP. GMC no. 2825328
Reviewed by Dr A. Wood, Dr C. Pugh, Dr B. Babor
Last reviewed 17 October 2022
Last updated 19 April 2024
Editorial policy
References
- NICE, 2021, Malaria prophylaxis - Assessment, accessed 08 September 2021
- BNF/NICE, 2017, Malaria, prophylaxis - Prophylaxis against malaria, accessed 08 September 2021
- Public Health England, 2021, Guidelines for malaria prevention in travellers from the UK 2021, accessed 08 September 2021
- World Health Organisation, 2021, Malaria, accessed 08 September 2021
- Jane Chiodini, 2021, Malaria Prevention, accessed 08 September 2021
- questions
- Choose
- order
Answer medical questions to order(antimalarials)
Honest & accurate responses are necessary for safe medical assessment
You must read the important medical information for malaria tablets.
- To find the malaria tablets recommended for the area you plan to visit please go to the NHS Fit for Travel website.
- On the NHS website find the page for the country you are visiting.
- On the top of your country page, click the link labelled 'Malaria Map'.
- On the malaria map page check if you are going to areas coloured red or pink and see the advice below the map. If antimalarials are usually advised return to the malaria section of the country.
- Your recommended tablets are listed on your country page under the heading 'Malaria precautions'. Make a note of ALL the recommended tablets.
Please take care to get this right as it is important. - People visiting multiple regions where different tablets are recommended must see their GP or a specialist travel adviser before ordering.
- If you are NOT eligible for treatment from Dr Fox consult your doctor or a specialist travel clinic. It is NOT safe to travel without appropriate malaria prevention.
The order process
Choose medication, register, and pay
Dr Fox issues prescription online
Pharmacy team post medication direct